
Newsroom
Certain species within the Achillea genus (Asteraceae family) have long been recognized for their medicinal value. Among the 10 Achillea species found in China—six of which are native to Xinjiang—Achillea millefolium (common yarrow) stands out as a globally used traditional remedy. It is employed across cultures to treat gastrointestinal disorders, liver and gallbladder ailments, skin inflammation, hypertension, respiratory infections, fever, and rheumatic conditions, among other issues.
A key source of yarrow's therapeutic potential lies in its sesquiterpenoids—naturally occurring compounds with diverse structures and potent biological activities, which have long been a focus of natural product chemistry research for their potential as drug leads.
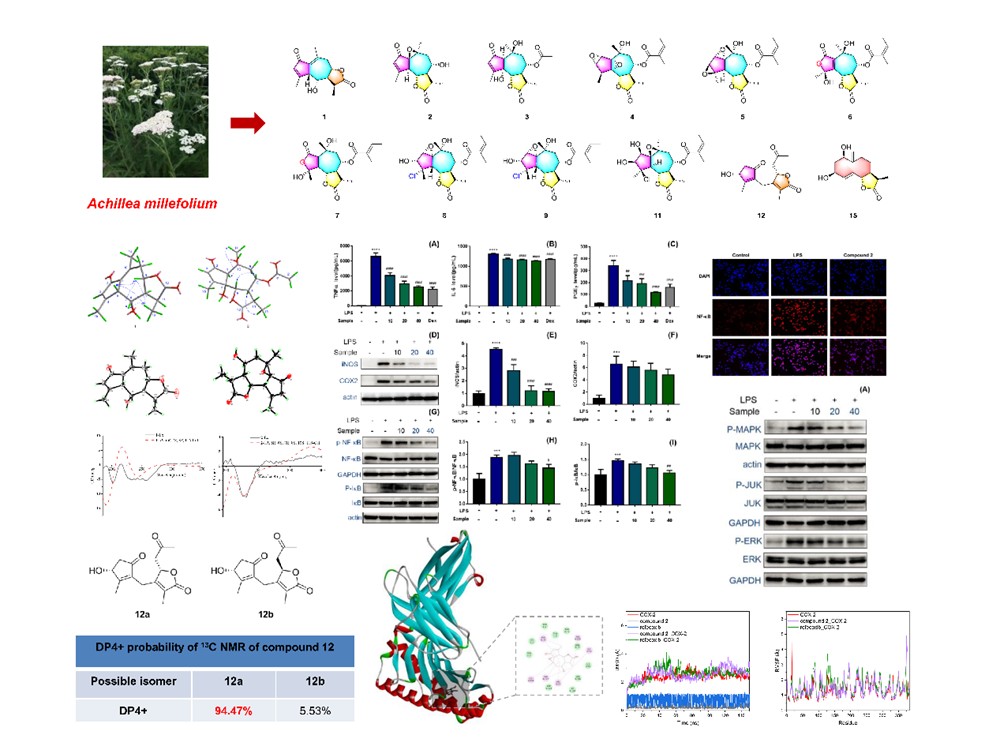
Now, a research team led by Prof. Haji Akber Aisa from the Xinjiang Technical Institute of Physics & Chemistry of the Chinese Academy of Sciences, has isolated 16 sesquiterpene lactones from the whole A. millefolium plant, including 12 previously unknown compounds. Their findings, published in the Journal of Molecular Structure, highlight the plant's rich chemical diversity.
The researchers used advanced spectroscopic techniques—including high-resolution mass spectrometry (HRMS) and nuclear magnetic resonance (NMR)—to determine the structures of the isolated compounds. Absolute configurations were confirmed via single-crystal X-ray crystallography, 13C NMR calculations paired with DP4+ probability analyses, and electronic circular dichroism (ECD) calculations.
Among the newly identified compounds, structural anomalies abounded: one pseudoguaianolide, two rare 3-oxa-guaianolides, three unusual chlorine-containing guaianolides, and one uncommon 1,10-seco-pseudoguaianolide. These discoveries underscore the remarkable structural variety of sesquiterpene lactones in A. millefolium, particularly among guaianolide-type compounds.
In subsequent tests, the team evaluated the compounds' anti-neuroinflammatory activity using a lipopolysaccharide (LPS)-induced BV2 microglial cell model. Five compounds were found to inhibit nitric oxide (NO) release, with one—dubbed millefoliumine S—exhibiting the strongest activity, with an IC50 value comparable to that of dexamethasone, a commonly used anti-inflammatory drug.
Further mechanistic studies on millefoliumine S revealed its potential to reduce neuroinflammation. Enzyme-linked immunosorbent assays (ELISA) showed it dose-dependently inhibited the release of pro-inflammatory mediators TNF-α, IL-6, and PGE2 in LPS-induced BV2 cells. Western blot and immunofluorescence analyses further demonstrated that millefoliumine S suppresses the expression of proteins linked to key inflammatory pathways, including iNOS/COX2, NF-κB, MAPK (JNK and ERK), and pyroptosis (NLRP3, cleaved-caspase 1, ASC, and IL-1β).
Molecular docking simulations provided additional insights: millefoliumine S formed stable hydrophobic interactions with target proteins via its seven-membered B ring and three methyl groups. Molecular dynamics studies confirmed its ability to bind stably to these proteins, supporting its potential as a therapeutic agent.
These findings validate the traditional use of A. millefolium for anti-inflammatory purposes and highlight sesquiterpene lactones—especially guaianolides—as key bioactive components. They also lay a foundation for developing natural lead compounds to treat inflammation-related diseases.
The research was supported by the Natural Science Foundation of Xinjiang Uygur Autonomous Region, and the Distinguished Young Scholars Program of Xinjiang Uygur Autonomous Region, among other sources.
Sesquiterpene lactones from Achillea millefolium L. and their anti-neuroinflammatory activity. (Image by Prof. Haji Akber Aisa's group)
